Abstract
Introduction & Objectives: Primary Cutaneous Follicle Center Lymphoma (PCFCL) is a very indolent mature B-cell lymphoma that shares germinal center morphology with follicular lymphoma (FL) but lacks the characteristic t(14;18). Unlike FL, immunohistochemistry fails to detect expression of BCL2, CD10, and immunoglobulin in PCFCL. Therefore, we investigated expression of B-cell receptor (BCR) transcripts to gain insight into the immunobiology of PCFCL.
Materials & Methods: Full-length heavy and light chain BCR transcripts of 13 histologically confirmed PCFCL were amplified using ARTISAN PCR and sequenced on the PacBio RSII system. BCR from 4 cases were sequenced to a depth of >2000 sequences per BCR transcript; the remaining cases to a median depth of 1663 sequences (range: 626-5301). BCR from 51 cases of FL and from peripheral B cells of 12 healthy donors were used as controls. Whole genome sequencing (WGS) and RNAseq were performed on 5 PCFCL on the Illumina HiSeq platform.
Results: No PCFCL case carried a t(14;18). In addition to previously described CD79B mutations, an L265P mutation in MYD88 was identified in one case, and two PCFCL carried amplifications in chromosome 2 involving the proto-oncogene REL.
ARTISAN PCR demonstrated expression of potentially functional VDJ and VJ genes with heavily mutated V regions (VDJ: 5.9-24.0%; VJ: 4.7-17.9%) in all PCFCL cases, which could be confirmed by RNAseq-based de novo BCR assembly. One PCFCL case expressed IgM, another IgA, and the remaining ten cases expressed IgG. PCFCL VDJ carried relatively long heavy chain CDR3 regions with a median of 19 amino acids (versus 17 in healthy donor PBMCs).
In contrast to FL, only minimal intraclonal sequence variation (comparable to the known error rate of the used sequencing method) was observed in PCFCL VDJ and VJ sequences, indicating absence of ongoing somatic hypermutation (SHM).
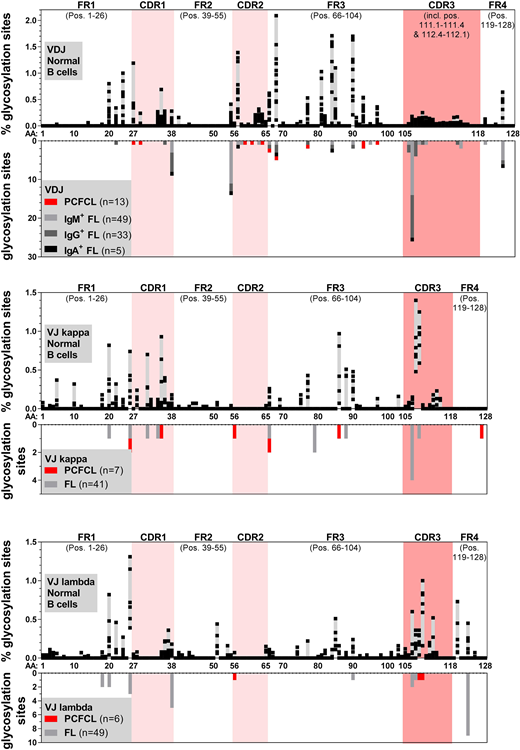
VDJ and/or VJ of 11 PCFCL (85%) carried at least one acquired N-linked glycosylation motifs, six PCFCL (46%) at least two, and one case four such motifs. 75% of acquired N-linked glycosylation motifs were found in different positions than the N-linked glycosylation motifs found in FL BCR (Figure). In contrast, only 17.5% and <0.5% of mutated BCR with less than 98% IGV homology to germ-line from healthy donors carried at least one or more acquired N-linked glycosylation motifs, respectively.
The PCFCL BCR with the most N-linked glycosylation motifs belonged to the only patient with a lymph node relapse and currently active disease. BCR of PCFCL with an at least partly follicular growth pattern appeared to carry more N-linked glycosylation sites than PCFCL with a strictly diffuse growth pattern (average 1.86 (range: 1-4) versus 0.83 (range: 0-2)), although this difference was not significant (p=0.10). Additionally, 6/7 PCFCL with a follicular growth pattern but none of the PCFCL with a diffuse growth pattern were situated on the scalp (p<0.001).
Conclusions: Follicular morphology, class switch recombination, and extensive SHM indicate a shared germinal center B cell origin for both PCFCL and FL. Clonal BCR sequences and previously identified copy number alterations prove that PCFCL represents a neoplastic clonal expansion. However, lack of ongoing SHM indicates that the immune follicles of PCFCL are not fully functional germinal centers. Since ongoing SHM is thought to contribute to lymphomagenesis by targeting non-BCR loci, absence of both ongoing SHM and the t(14;18) may explain the relatively benign clinical course of PCFCL compared to FL. As previously described for FL, continuous BCR stimulation through glycosylation-mediated binding of lectins on resident cells of the follicular microenvironment may play a role in the clonal expansion of PCFCL cells and may orchestrate the follicular microarchitecture in PCFCL. However, the positions of the glycosylated amino acids within the BCR vary between FL and PCFCL and warrant functional studies to clarify their relevance in PCFCL pathogenesis.
Schmidt:Gilead: Honoraria, Other: Travel Grants; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal